
Just follow these steps to get the exact results for substances: The online specific heat capacity calculator is helps you to find heat capacity of different substances. This is the typical heat capacity of water and it can be calculated by specific heat calculator as well in one go. Just put the values in specific heat equationas\( c = Q / (m x ΔT)\). Let’s suppose the difference is \(ΔT = -3 K\) and m is 5 kg. Now decide the difference of between the initial and final state of the sample. For instance, say that we want to decrease the taster’s thermal energy by \(63,000 J\). While cooling down the sample, you have to give the subtracted energy as a negative value. Now give the amount of energy supplied as a positive value. Have a look down below and follow some simple steps:įirst of all, you have to Determine whether you want to warm up the substance or cool it down. With the support of specific heat formula calculation of specific heat is and easy process.
#Heat equation chemistry calculator manual#
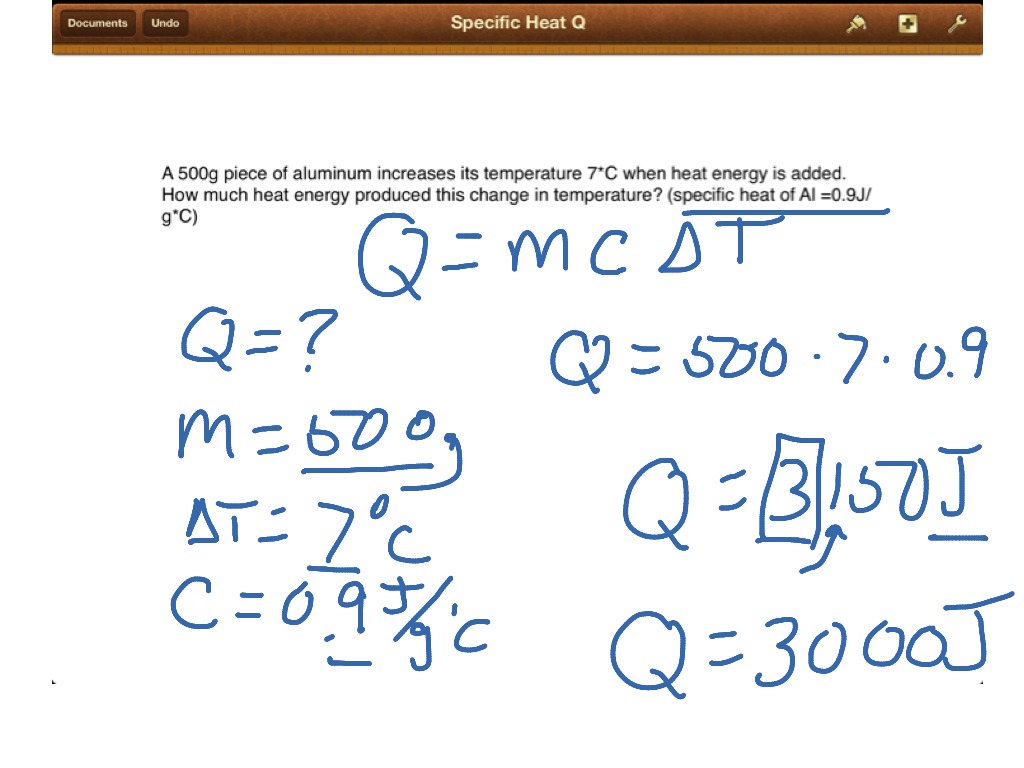
However, a specific heat calculator can assist you in finding the values without any hustle of manual calculations. To find specific heat put the values in above specific heat equation: \(\frac = 0.231\). How will you calculate its specific heat? If you have a \(15 g\) piece of any metal that absorbs \(134 J\) of heat while increasing from \(24.0☌\) to \(62.7☌\). \(☌\) is degrees centigrade or Celsius.\(\Delta T\) is the temperature difference.\(Q\) is representing the induced thermal energy.\(C\) is representing the specific heat capacity.in case of water it shouldn’t get boiled.

These phenomena should take place within a temperature range, where the substance doesn’t change its state e.g. To find specific heat capacity we can say that it is a measure of the total energy that is needed to heat up 1 kilogram of any material to 1☌elcius or 1Kelvin. It is the amount of heat that is required to change the temperature of a mass unit of any substanceonly by one degree. When it comes to analyze specific heat of water or any other substance, it tells us the specific heat formula along with whole solution for a respective substance. The specific heat calculator helps to find the specific heat, heat energy, mass of substance, initial temperature, and final temperature of any substance.


 0 kommentar(er)
0 kommentar(er)
